
Bromine Facts, Symbol, Discovery, Properties, Uses
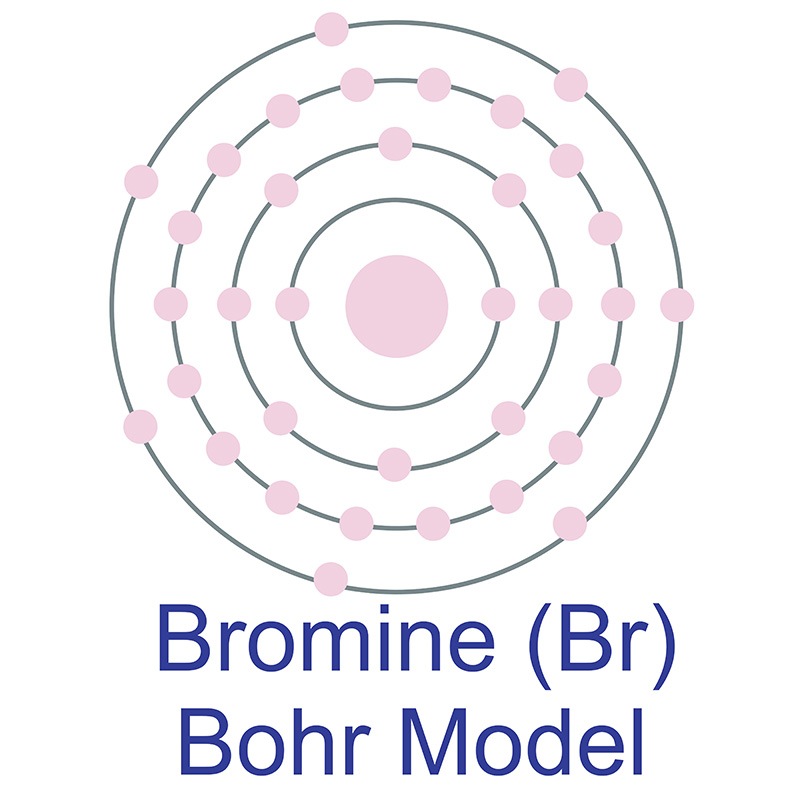
Bromine (Br) electron configuration (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle. Bromine atom electron configuration through orbit Scientist Niels Bohr was the first to give an idea of the atom's orbit.

Bromine (Br) Properties & Uses StudiousGuy
Bohr model is a structural model in which the negatively charged electrons revolve around the positively charged nucleus. This is similar to the planets revolving around the sun, except that the orbits are non-planar. The electrons move in a fixed orbits (shells) and each orbit has a fixed energy. Each orbit (or shell) can hold a certain number.

Bohr Model Bromine
Figure \(\PageIndex{7}\) In Bohr's Model of the atom, electrons absorb energy to move to a higher level and release energy to move to lower levels. (CC BY-SA 3.0; Kurzon). The evidence used to support Bohr's model came from the atomic spectra. He suggested that an atomic spectrum is made by the electrons in an atom moving energy levels.

7+ bromine bohr diagram RajibRajisha
In atomic physics, the Bohr model or Rutherford-Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons.

70+ Bromine Gas Stock Photos, Pictures & RoyaltyFree Images iStock
0:00 / 3:51 Bohr-Rutherford Diagram for Bromine (Br) chemistNATE 251K subscribers Subscribe 230 17K views 3 years ago Bromine has 18 electrons in its third shell because it is past Zinc on the.

Valence electron, Electron shell, Bromine, bromide, Electron
From the Bohr model, it can be found that the number of orbits or shells in bromine is 4. Hence, as bromine has 4 orbits, it lies in period 4 of the Periodic table. Why is Bromine in p-block? Before knowing this reason, first of all I want to ask you a simple question. How can you determine the blocks-wise position of elements?
Bromine Bohr Diagram
In 1913, a Danish physicist, Niels Bohr (1885-1962; Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Bohr's model required only one assumption: The electron moves around the nucleus in circular orbits that can have only certain allowed radii.
Bohr Model Bromine Atom Electron Structure เวกเตอร์สต็อก (ปลอดค่า
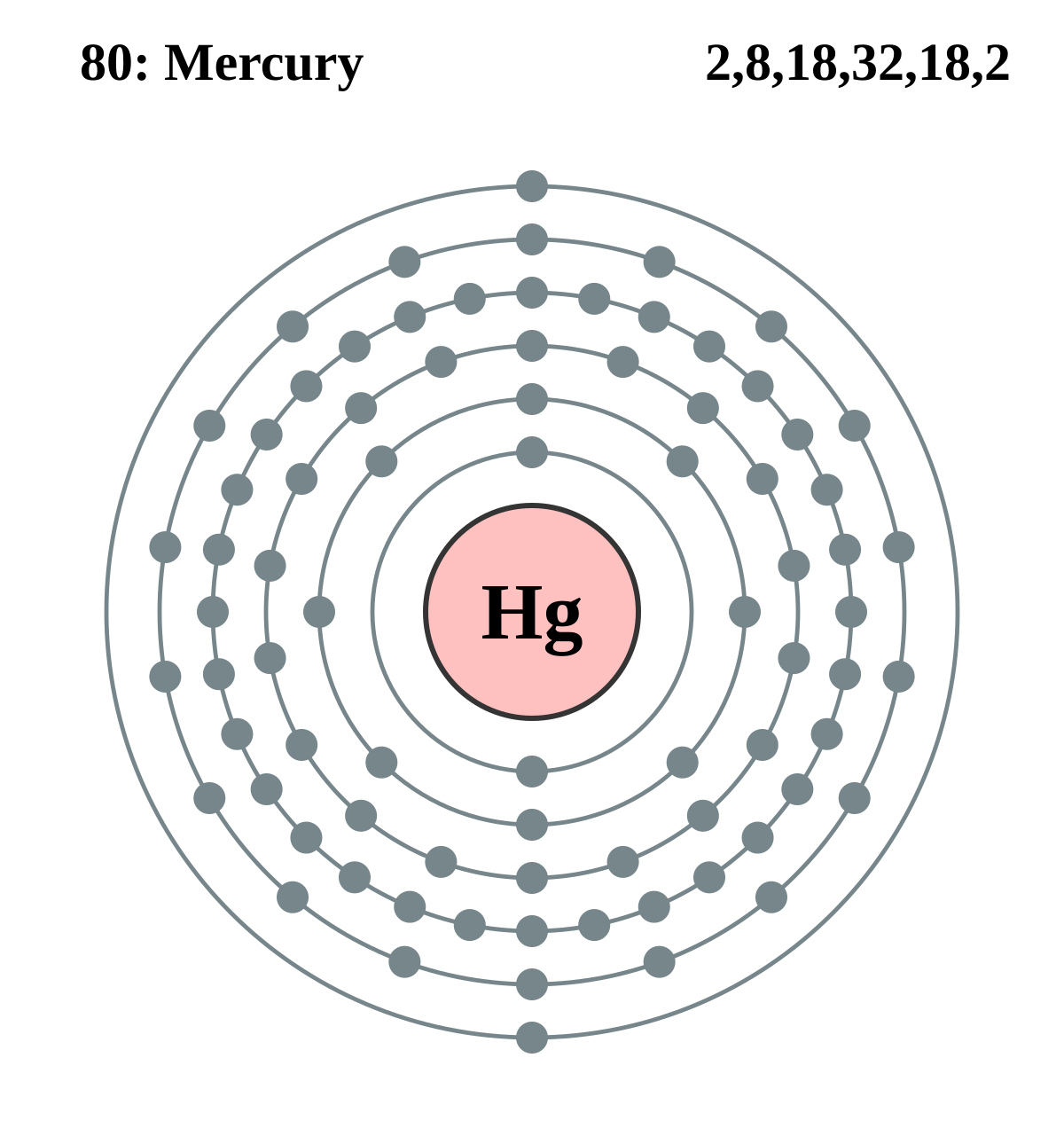
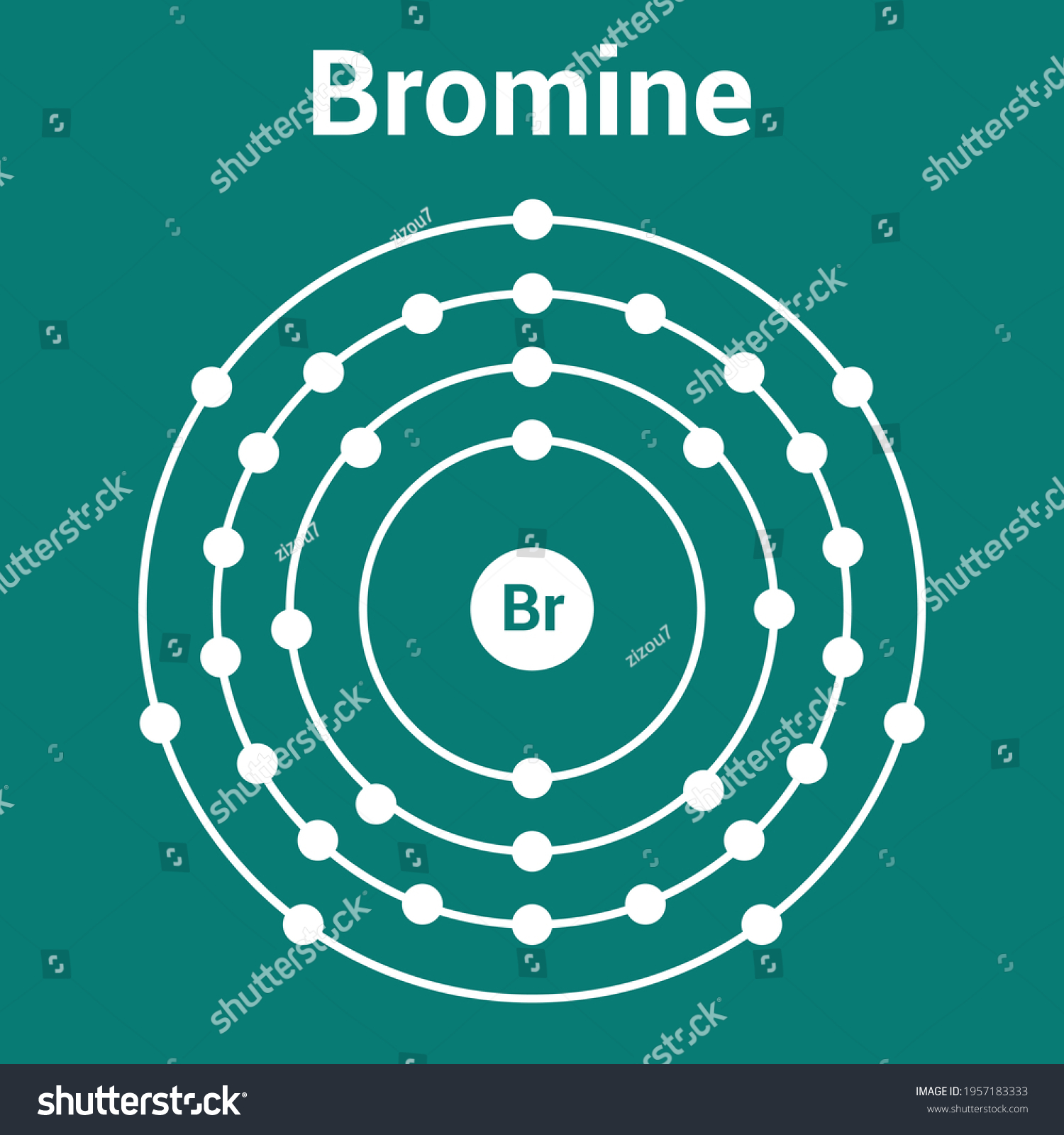
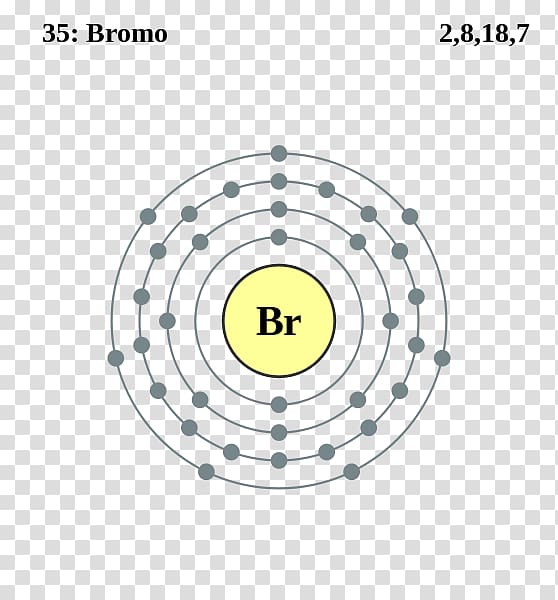
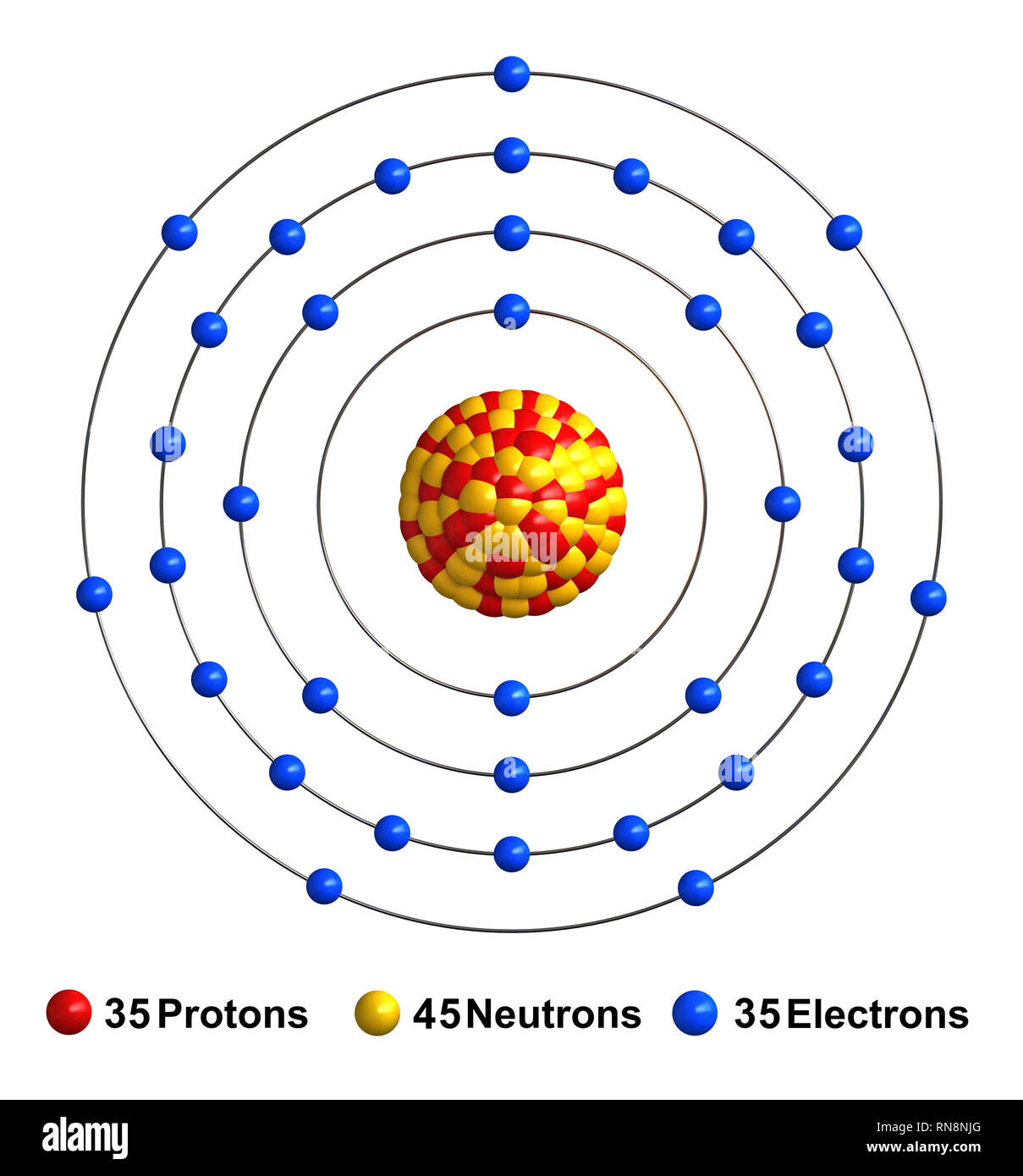
The Bohr Model of Bromine (Br) has a nucleus that contains 45 neutrons and 35 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Bromine contains 7 electrons that also called valence electrons. Page Contents show How to draw Bohr Model of Bromine (Br)?
Electron Configuration Bromine Chemical Element Electron Shell Bohr
The Bohr Model of Bromine (Br) has a nucleus with 45 neutrons and 35 protons. This nucleus is surrounded by four electron shells. The first shell of the Bohr diagram of Bromine has 2 electrons, the 2nd shell has 8, the 3rd shell has 18, and the 4th shell has 7 electrons. Also check - How to draw Bohr model diagram for an atom?

Bromine Bohr Diagram
Basic Information Name: Bromine Symbol: Br Atomic Number: 35 Atomic Mass: 79.904 amu Melting Point: -7.2 °C (265.95 K, 19.04 °F) Boiling Point: 58.78 °C (331.93 K, 137.804 °F) Number of Protons/Electrons: 35 Number of Neutrons: 45 Classification: Halogen Crystal Structure: Orthorhombic Density @ 293 K: 3.119 g/cm 3 Color: Red Atomic Structure

Bohr Model Bromine Atom Electron Structure Stock Vector (Royalty Free
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
Bromine (Br) AMERICAN ELEMENTS
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?
Bromine (Br) AMERICAN ELEMENTS
This game presents a simple introduction to the Rutherford-Bohr model of the atom and the way we organize the elements. For this game, the most common isotopes of the chemical elements are used. General questions about the properties of elements assume standard temperature and pressure (helium is liquid below -268°C and gold is a liquid above.

How Can We Find A Electron Configuration For Bromine (Br)
Bohr Atomic Model of Bromine - Electrons per energy level. n s p d f; Ground State Electronic Configuration of Bromine - neutral Bromine atomAbbreviated electronic configuration of . BromineThe ground state abbreviated electronic configuration of Neutral . Bromine atom is [Ar] 3d10 4s2 4p5.
Numero De Electrones De Bromo lios
#1 Using aufbau principle #2 Using periodic table #3 From its Bohr model #4 From its orbital diagram Let's break down each method in detail. Using aufbau principle First, find electrons of bromine atom Periodic table The atomic number of bromine represents the total number of electrons of bromine.
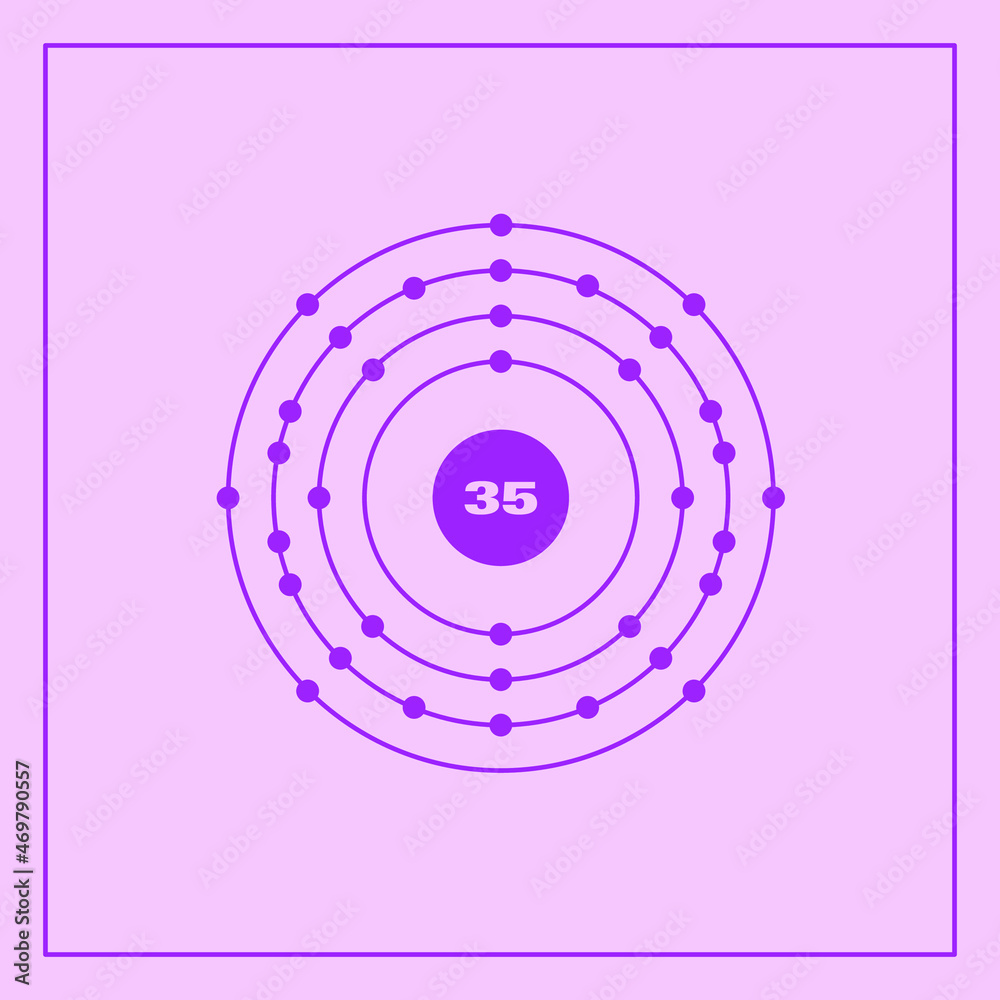
Bohr model representation of the bromine atom, number 35 and symbol Br
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or 'shells', around the central nucleus. This is sometimes called the Bohr, or the 'solar system', model.